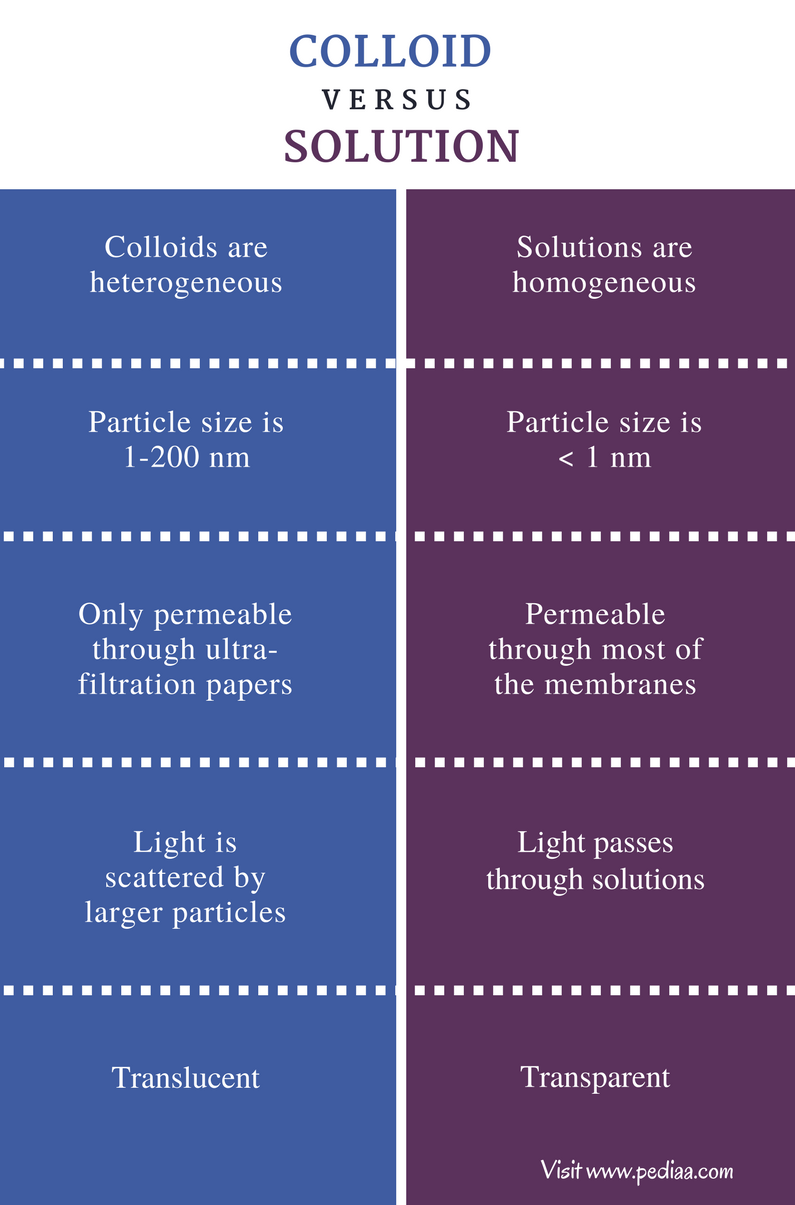
Solutions, Suspensions, and Colloids Suspensions and colloids are special types of liquids often studied with solutions. Both are commonly mistaken for solutions, but. Active Chemistry Movie Special Effects Active Chemistry 122 CLASSIFYING MIXTURES In this activity you mixed together water and several different materials to produce different kinds of mixtures. Based on the nature of interaction between the dispersed phase and the dispersion medium, colloids can be classified as: Hydrophilic colloids: The colloid particles are attracted toward water. They are also called reversible sols. Start studying Suspensions, Colloids, and Solutions. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Solutions and suspensions are both items that are mixtures of two or more components. A solution mixes thoroughly and is usually clear, whereas a suspension doesnt mix thoroughly, and it appears cloudy in color. After suspensions sit for quite some time, the components tend to separate. Both crystalloids and colloids increase intestinal blood flow and systemic arterial pressure; however, colloids may have a longer duration of effect. Colloids also result in a net movement of fluid from the intestinal lumen to the blood, whereas crystalloids can exacerbate transmucosal fluid. Start studying differences between solutions, colliods, and suspensions. Learn vocabulary, terms, and more with flashcards, games, and other study tools. The difference between mixtures, solutions and suspensions. Mixtures, Solutions and Suspensions The difference between mixtures, solutions and suspensions. A mixture is a combination of substances which are not chemically joined together. The main difference between a colloid and a suspension is that a suspension will separate into particles, but a colloid will not. A colloid is the middle line between a suspension and a solution. A suspension is composed of at least two substances that are visible in the suspension. Suspensions, Colloids and Solutions. The difference between Molarity and Molality. Suspensions, colloids and solutions. And actually, I'll put a little contest on this video, if you all can think of good ways to remember the difference between molality and molarity. Suspensions, colloids and solutions. The difference between molarity and molality. Suspensions, colloids and solutions are all different kinds of mixtures comprising at least two components, let's call them A and B. The difference Suspensions separate, colloids form lumps and may look 'cloudlike' and solutions remain the same. What are the differences between a solution suspension and a colloid. Colloids can be distinguished from solutions using Tyndall effect. Tyndall effect is defined as the scattering of light (light beam) through a colloidal solution. The particles are termed as colloidal particles and the mixture formed is known as colloidal dispersion. Suspensions may scatter light if conditions are right. A colloid is a mixture with intermediatesized particles. With colloids, the particles are not easily seen, and the particles will not settle. Within the categories of homogeneous and heterogeneous mixtures there are more specific types of mixtures including solutions, alloys, suspensions, and colloids. Solutions (homogeneous) A solution is a mixture where one of the substances dissolves in the other. Mixture is an association of several substances. Suspensions, solutions, and colloids are examples of two such mixtures. Since the components in a mixture are not chemically bound together, they can be physically separated by filtration, precipitation, evaporation, etc. What is the difference between solutions suspensions and Colloids? If a mixture settles over time and separates it is a suspension (milk with chocolate added). If a mixture does not separate overtime but forms lumpy or fluffy masses (like cot tage cheese) it is a colloid. A suspension is a heterogeneous mixture of solid particle in carrier liquid. It is not dissolved it is suspended. The emulsion is the mixture of two or more immiscible liquids. A colloidal solution is the mixture of sub micronic particles in carrier liquid. Colloids can be distinguished from solutions using the Tyndall effect. Light passing through a colloidal dispersion, such as smoky or foggy air, will be reflected by. The main difference between colloid and solution is the size of their particles. Particles in solutions are tinier than that of colloids. Solute particles are not visible under a light microscope; however, colloid particles can be seen under the same. Particles intermediate in size between those found in solutions and suspensions can be mixed such that they remain evenly distributed without settling out. These particles range in size from 10 8 to 10 6 m in size and are termed colloidal particles or colloids. The difference between a solution and a suspension is in the particle sizes involved. A solution is a mixture of ions or molecules (very, very small). Colloids can be distinguished from solutions using Tyndall effect which is the scattering of light through a colloidal solution. The particles are termed as colloidal particles and the mixture formed is known as colloidal dispersion formed by mixture of liquid, solid and gases. Similarities and Differences Between Solutions and Colloids One similarity is that nether of their particles settle. One difference is a solution is in the liquid or gas state, but a colloid is a. The main difference between colloid and suspension lies in the size of particles. Colloid particles are much smaller than suspension particles. Due to this size difference, colloid particles can be either homogeneous or heterogeneous at given conditions, whereas suspensions are always heterogeneous. Difference between Solutions, Suspensions, and Colloids Solution is a mixture of two or more substances in a single phase. At least two substances must be mixed in order to have a solution. DOWNLOAD DIFFERENCE BETWEEN COLLOIDS AND SOLUTIONS difference between colloids and pdf Based on the nature of interaction between the dispersed phase and the. Both are types of solutions, the only difference is the size of the particles. Suspensions are made up of large particles eg. sand, while colloids are smaller particles eg. The basic difference between a colloid and a suspension is the diameter of the particles dispersed. Colloids are gen erally 1 to 5 nanometers while suspensions are usually 1000 nanometers. Both are examples of solutions. On the other hand, a suspension is inhomogeneous. Typically this is a slurry of finely divided solid in water or some other solvent, from which the solid has precipitated. Suspensions, Colloids and Solu Suspensions, Colloids and Solutions. Log in to save your progress and obtain a certificate in Alisons free Chemistry The Nature of Substances online course. Log in with your Social Account. Suspension vs Solution Chemistry is the physical science which deals with matter and the changes that it goes through during chemical reactions. It deals with the chemical reaction between substances that are mixed together and how they are transformed into another substance. Solutions and suspensions are mixtures of Knowledge application use your knowledge to answer questions about the particles contained in colloids Distinguishing differences compare and contrast the difference between a solution and a. Solutions, Suspensions and Emulsions Liquid mixtures can be divided into 4 main types: solutions, suspensions, colloids and emulsions Solutions: consist of soluble material or material (solute) dissolved in a liquid (solvent)are clearare homogenous (one phase) and do not settle. Colloidal Solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i. Smoke from a fire is example of colloidal system in which tiny particles of solid float in air. The colloids are relatively homogenous, while the suspensions are heterogeneous. The colloid particles can pass through filter paper, while the particles of the suspensions cannot. Examples of colloids are gelatin in water, starch in water, sodium chloride in benzene, etc. Examples of suspensions are sand in water, powdered chalk in water. Colloids are particles smaller than those in a suspension. The basic difference between a colloid and a suspension is the diameter of the particles dispersed. Colloids are generally 1 to 5 nanometers while suspensions are usually 1000 nanometers. solution vs colloid Mixture is a collection of different substances, which are physically combined, but do not join chemically. Mixtures show different physical or chemical properties than the individual substances. Solutions and colloids are two such mixtures with different properties. In these mixtures, solid, gaseous or liquid substances are mixed in different ratios. DOWNLOAD DIFFERENCE BETWEEN COLLOIDS AND SOLUTIONS difference between colloids and pdf 1I INTRODUCTION 1 II TABLE OF CONTENTS 2 III BASIC PRINCIPLES OF COLLOID SCIENCE 6 III1 Colloidal suspensions 6 III11 Hydrophobic colloids 6 Water Soluble Polymers snf. au A solution is a mixture featuring solutes that have been dissolved, while a suspension is a mixture of liquids also containing solid particles that may not completely dissolve inside the liquid. Materials that dissolve in liquids are considered soluble. When no more solute dissolves in a particular. Colloidal Silver What They Don't Tell You and What You Need To Know Earth Clinic Duration: 3: 56. Earth Clinic 204, 268 views For more on Mixtures (Solutions, Suspensions, Emulsions, Colloids). A suspension is a mixture between two substances, one of which is finely divided and dispersed in the other. Common suspensions include sand in water, dust in air, and droplets of oil in air. Particles in a suspension are larger than those in a solutions; they are visible under a microscope and can often be. A colloid is a type of mixture intermediate between a homogeneous mixture (also called a solution) and a heterogeneous mixture with properties also intermediate between the two. The particles in a colloid can be solid, liquid or bubbles of gas. A colloid is intermediate between a solution and a suspension. While a suspension will separate out a colloid will not. While a suspension will separate out a colloid will not. Colloids can be distinguished from solutions using the Tyndall effect. A colloid is a substance microscopically dispersed throughout another substance. Some colloids are translucent because of the Tyndall effect, which is the scattering of light by particles in the colloid. Other colloids may be opaque or have a slight color. Milk is an emulsified colloid of liquid.