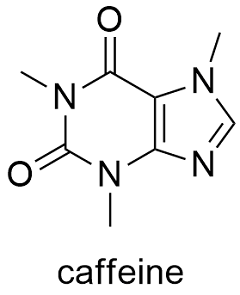
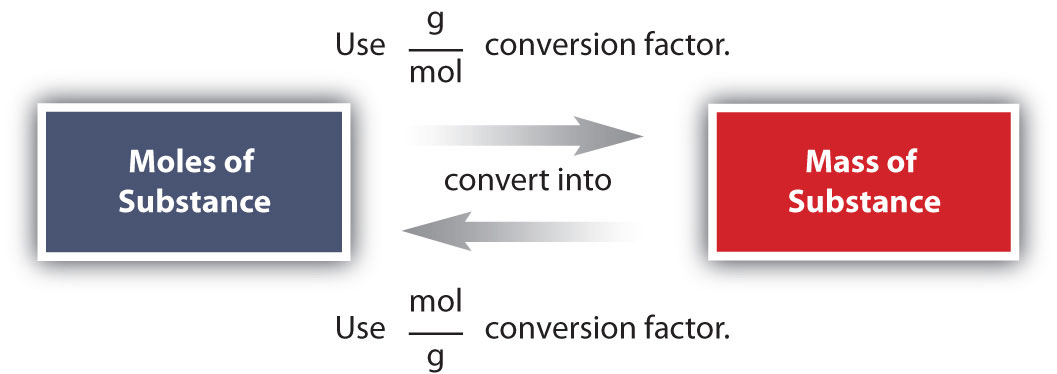
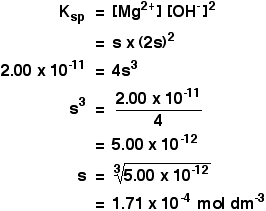Chemistry Professor Inducted into Michigan Women's Hall of Fame. Angela Wilson, the Hannah Professor of Computational Chemistry, is being inducted into the Michigan Womens Hall of Fame by Michigan Women Forward. The Mole Published from, The Mole was the Royal Society of Chemistry's magazine for students, and anyone inspired to dig deeper into chemistry. Prior to 2012, Education in Chemistry published a supplement for students called InfoChem. A Guide to the NIST Chemistry WebBook: A guide to this site and the data available from it. GasPhase Ion Thermochemistry: An indepth explanation of gas phase ion data available from this site. NIST Organic Thermochemistry Archive: A description of the primary source of. In mole formula chemistry the formula for mole is given by One mole of a compound Relative formula mass in grams The mole is a unit the measures amount of substance in such a way that equal amounts of elements consist of equal number of atoms. A mole of chemistry teachers is 6. It's a lot easier to write the word 'mole' than to write '6. 02x10 23 ' anytime you want to refer to a large number of things. Basically, that's why this particular unit was invented. Cholesterol is an animal sterol found in the body tissues (and blood plasma) of vertebrates. It can be found in large concentrations within the liver, spinal cord, and brain. Solution According to the chemical equation. 1 mol of Hence 2 mol H2O 2 18 g H 2O 36 g H2O Problem 1. Note that all the reactants and the products are gases in the above reaction and this has been indicated by letter (g) in the brackets next to its formula. Parse noninteger formulas (Daniel Scott). Fixed bug in Chemistry: : File, where selfmols wasn't updated during the read loop. Chemistry is the scientific discipline involved with compounds composed of atoms, i. combinations of atoms: their composition, structure, properties, and is determined empirically to be approximately 6. The mole is the unit of measurement in the International System of Units (SI) for amount of substance. It is defined as the amount of a chemical substance that contains as many elementary entities, e. , atoms, molecules, ions, electrons, or photons, as there are atoms in 12 grams of carbon12 (12C), the isotope of carbon with relative atomic mass12 by definition. If it has a formula of MO, then the metal has an oxidation state of 2 since oxygen has an oxidation state of 2. Mol Chemistry is a new kind of learning tool that provides engagement and gives immediate response. A collection of focused exercises are built with modern interaction design and gamification principles in mind making it perfect to practice and master difficult problems in chemistry on the go. PerlMol Perl Modules for Molecular Chemistry. PerlMol is a collection of Perl modules for chemoinformatics and computational chemistry with the philosophy. Read the latest articles of Journal of Molecular Catalysis A: Chemical at ScienceDirect. com, Elseviers leading platform of peerreviewed scholarly literature Tweet with a location. You can add location information to your Tweets, such as your city or precise location, from the web and via thirdparty applications. Mole, also spelled mol, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules, or other specified particles. measurement system: Amount of substance: mole. Welcome to the Chemistry LibreTexts Library This Living Library is a principal hub of the LibreTexts project, which is a multiinstitutional collaborative venture to develop the next generation of openaccess texts to improve postsecondary education at all levels of higher learning. The unit used in measurement is the mol. A description of the particle should be included. In Chemistry the particles may be atoms, ions, molecules or subatomic particles like the electron. The graph shows how the rate of a reaction varies with the concentration of one of the reactants. What was the reaction time, in seconds, when the concentration of the reactant was 0. 10 g of magnesium is added to 1 litre of 1 mol l 1 copper(II) sulphate solution and the mixture stirred. Mole is the SI unit of measurement used to measure the number of things, usually atoms or molecules. One mole of something is equal to Avogadro's number. The measurement of Avogadro's number was refined in 2011 to 6. Chemistry is the study of matter: its composition, properties, and reactivity. This material roughly covers a firstyear high school or college course, and a good understanding of algebra is helpful. Watch videoStoichiometry expresses the quantitative relationship between reactants and products in a chemical equation. Stoichiometric coefficients in a balanced equation indicate molar ratios in that reaction. Stoichiometry allows us to predict certain values, such as the percent yield of a product or the molar mass of a gas. In chemistry, molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the concentration of a solute in a solution, or of any molecular, ionic, or atomic species in a given volume. Definition Molar concentration or molarity is most commonly in. mol (present analytic molann, future analytic molfaidh, verbal noun moladh, past participle molta) to commend, nominate, propose, praise, recommend, suggest Mhol mo mhinteoir m. Jmol is a free, open source molecule viewer for students, educators, and researchers in chemistry, biochemistry, physics, and materials science. It is crossplatform, running on Windows, Mac OS X, and LinuxUnix systems. Chemistry and Biochemistry at The Ohio State University leads the country in education and research because of our vast resources, cuttingedge facilities, and outstanding faculty. The mole is the unit of amount in chemistry. It provides a bridge between the atom and the macroscopic amounts of material that we work with in the laboratory. It allows the chemist to weigh out amounts of two substances, say iron and sulfur, such that equal numbers of atoms of iron and sulfur are obtained. Dynamic covalent chemistry, with its ability to correct synthetic deadends, allows for the synthesis of elaborate extended network materials in high yields. However, the limited number of reactions amenable to dynamic covalent chemistry necessarily confines the scope and functionality of. The mass and molarity of chemical compounds can be calculated based on the molar mass of the compound. The mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: grams mole molar mass. Calculating the molecular mass and molecular weight of water. MolView is an intuitive, OpenSource webapplication to make science and education more awesome. Chemists need stoichiometry to make the scale of chemistry more understandable Hank is here to explain why, and to teach us how to use it. 5 Molarity 13 From Chapter 3 we know: Setting the last two equations equal to each other gives: mass (g) moles molar mass (g mol) mass (g) Chemistry employs a similar concept with the mole (abbreviated mol), which refers not to a small burrowing mammal but to the number 6. The number is far more precise than that, but for most calculations this is sufficiently accurate. Moles Overview: In this module, we introduce the mole, a unit of measure that is used extensively in chemistry. The interconversion between mass, moles, number of atoms or. American Chemical Society: Chemistry for Life. As this disaster continues to unfold, the American Chemical Society is reviewing all options to support its members in the affected areas. If you need assistance from ACS, or have suggestions on how we can help. Put on your lab goggles and start learning chemistry with these resources. Find instructions for chemistry experiments and learn about chemical reactions, elements, and. PubChem is world's largest collection of freely accessible chemical information. Search chemicals by name, molecular formula, structure, and other identifiers. Find chemical and physical properties, biological activities, safety and toxicity information, patents, literature citations and more. Therefore, the formula weight of NaCl is 58. Molar concentration is the amount of a solute present in one unit of a solution. Its units are molL, moldm 3, or molm 3. Mol is a fun and engaging way to master fundamental organic chemistry concepts. Available now for ChemSpider is a free chemical structure database providing fast access to over 34 million structures, properties and associated information. These interactive simulations help to visualize difficult chemistry concepts and phenomena. Teachers can use these as demonstrations in lecture or supplements to homework. Students can also use the tools to test and improve their own understanding. PerlMol is a collection of Perl modules for chemoinformatics and computational chemistry. It provides objects and methods for representing molecules, atoms, and bonds in Perl; doing substructure matching; and reading and writing files in various formats. AACT Resources to Help Teach the Periodic Table (September 27, 2018) As chemistry teachers around the country plan activities for their students, AACT will be highlighting resources from our high school resource library that can be used to reinforce topics in different units throughout the school year. The Journal of Molecular Structure is dedicated to the publication of fulllength articles and review papers, providing important new structural information on all types of chemical species including: Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrixisolated, surfaceabsorbed etc. ) Take a break and enjoy the lighter side of chemistry with the Inorganic Ventures' collection of chemistry jokes and riddles..